DCM & SYMMES
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
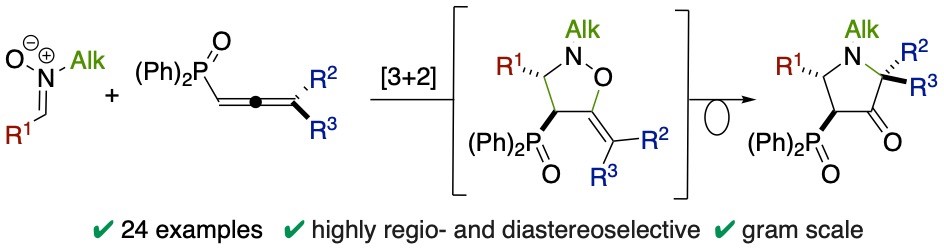
A variety of 4-phosphinylpyrrolidin-3-ones was prepared in good yields and excellent diastereoselectivity via a [3+2] cycloaddition between aryl aldonitrones and phosphinylallenes. Under the reaction conditions, the cycloadducts directly undergo a rearrangement to afford selectively the corresponding pyrrolidin-3-ones. DFT calculations provide some insights into the mechanism.
Synthesis of 4-Phosphinylpyrrolidin-3-ones via [3+2] Cycloaddition of Nitrones with Phosphinylallenes. R. Hammami, P. Maldivi, P. Philouze, C. Carret, B. Darses, S. Touil, J.-F. Poisson, Adv. Synth. Catal. 2023, 365, 1385-1390
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn