- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
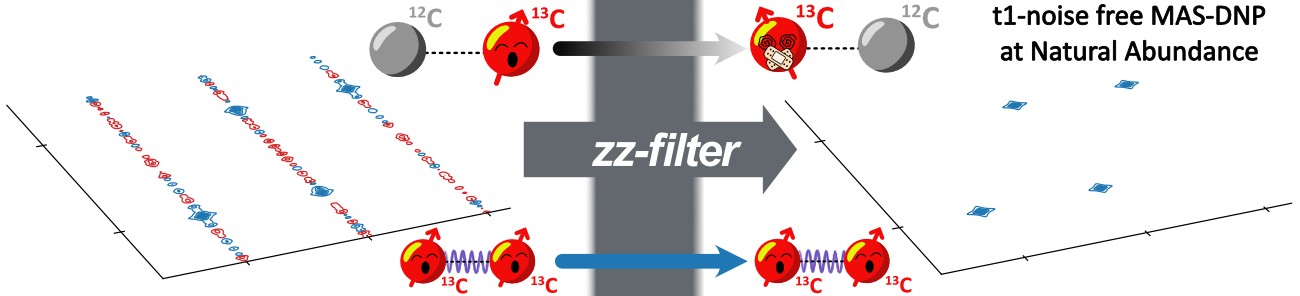
Dynamic Nuclear Polarization (DNP) has greatly increased solid-state NMR sensitivity, enabling multidimensional correlation experiments (e.g., 13C–13C, 13C–15N) at natural isotopic abundance. Yet, such experiments often suffer from t1-noise, an artefact that obscures weak cross-peaks. Here, we present a method to suppress t1-noise in 13C–13C correlation spectra. This so-called zz-filter markedly improves the spectral quality on both commercial (100 K) and custom-built helium-spinning (30 K) DNP set-ups, with up to a fivefold increase in signal-to-noise ratio in the indirect experimental dimension.
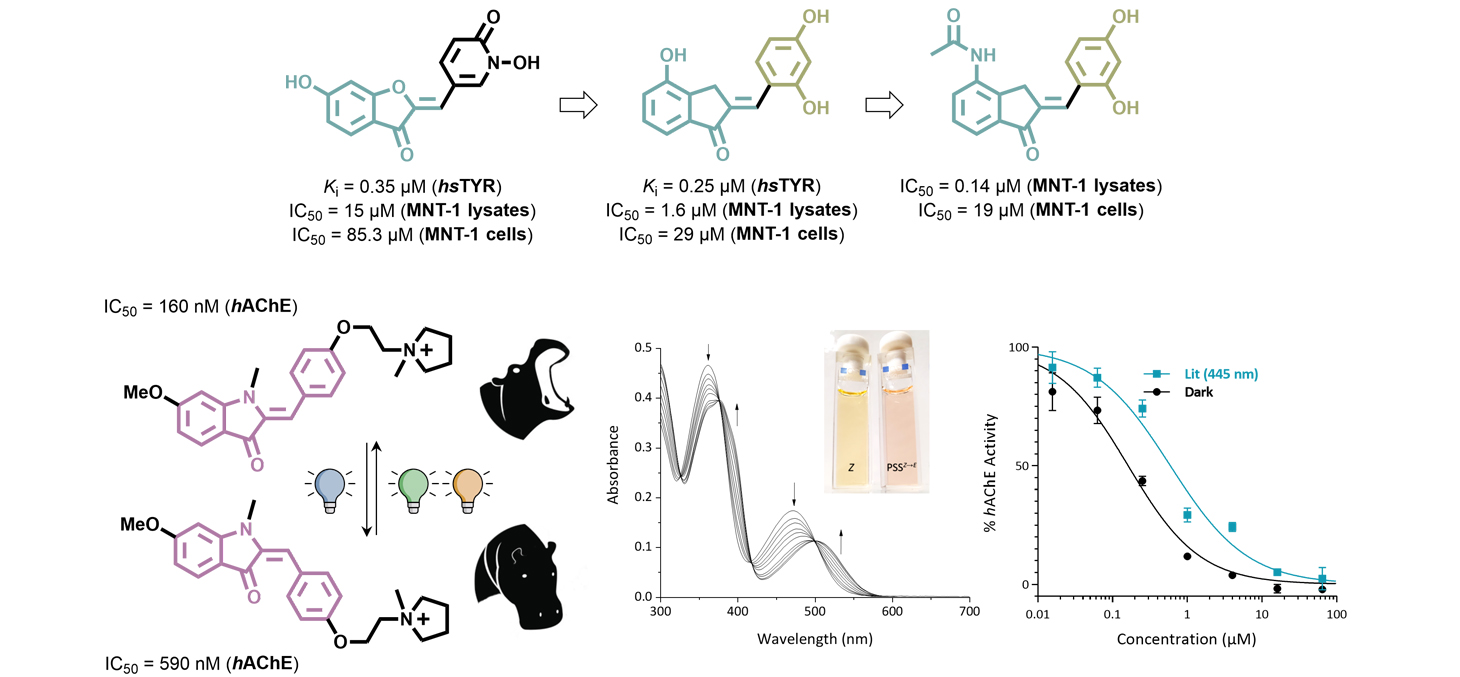
The project enabled the development of new series of hemi-indigoid compounds targeting two distinct applications. In the first line of research, three successive generations of human tyrosinase inhibitors were synthesized, yielding over one hun-dred distinct compounds. These inhibitors demonstrated sub-micromolar activity and exhibited enhanced efficacy in cellular models, highlighting their potential for therapeutic applica-tions. In the second line of work, eight photoswitchable hemi-indigoid derivatives were designed and synthesized as the first reported photopharmacological compounds of this class. These compounds functioned as acetylcholinesterase inhibitors, of-fering a novel approach for visible-light-controlled modulation of enzyme activity. Together, these efforts underscore the versatility of hemiindigoid scaffolds for both enzymatic inhibi-tion and photopharmacological applications.
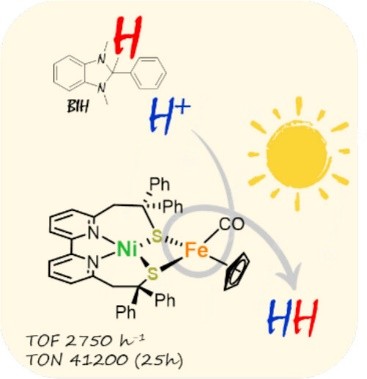
Developing efficient photocatalytic systems for sustainable H2 production is a crucial step toward reducing dependence on fossil fuels. While extensive research over the past two decades has focused on bioinspired FeFe catalysts, NiFe-based systems remain largely unexplored. This study addresses that gap by introducing the first heterodinuclear NiFe complex capable of catalyzing H2 production under photoassisted conditions. The catalyst demonstrates remarkable performance, achieving a turnover frequency of 2750 h–1 and a turnover number of up to 41200. A comprehensive mechanistic investigation, supported by EPR and labeling experiments, reveals that the photodriven catalytic pathway differs from the electrocatalytic mechanism. Under photoassisted conditions, BIH not only serves as a sacrificial electron donor, but its oxidized form, BIH·+, also acts as a hydrogen atom donor, directly contributing to the high activity of this system. These findings highlight the dual role of BIH in photoassisted catalysis, extending beyond its specific application in H2 production to other reactions of interest, such as CO2 reduction.
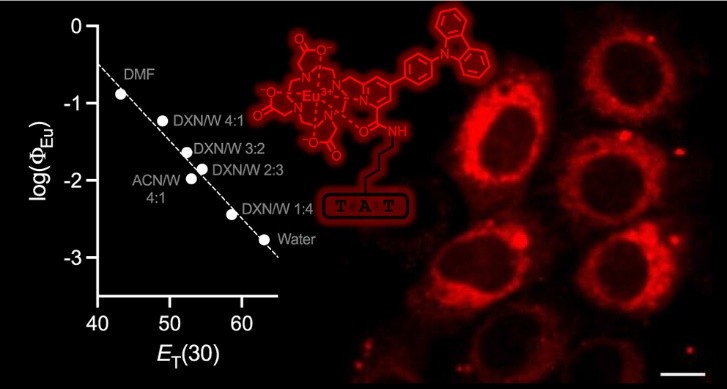
Lanthanide(III) complexes with two-photon absorbing antennas are attractive for microscopy imaging of live cells because they can be excited in the NIR. We describe the synthesis and luminescence and imaging properties of two Eu3+ complexes, mTAT[Eu·L-CC-Ar–Cz] and mTAT[Eu·L-Ar–Cz], with (N-carbazolyl)-aryl-alkynyl-picolinamide and (N-carbazolyl)-aryl-picolinamide antennas, respectively, conjugated to the TAT cell-penetrating peptides. Contrary to what was previously observed with related Eu3+ complexes with carbazole-based antennas in a mixture of water and organic solvents, these two complexes show very low emission quantum yield (ΦEu < 0.002) in purely aqueous buffers. A detailed spectroscopic study on mTAT[Eu·L-Ar–Cz] reveals that the quantum yield of emission is strongly polarity dependent─the less polar the medium, the higher the quantum yield─and that the emission quenching in water is likely due to a photoinduced electron transfer between the excited carbazole-based antenna and Eu3+ that efficiently competes with the energy transfer process. Nevertheless, mTAT[Eu·L-Ar–Cz] shows a significant two-photon cross-section of 100 GM at 750 nm, which is interesting for two-photon microscopy. The live cell imaging properties of mTAT[Eu·L-Ar–Cz] and the two other conjugates were investigated. Cytosolic delivery was clearly evidenced in the case of mTAT[Eu·L-Ar–Cz] when cells are coincubated with this compound and a nonluminescent dimeric TAT derivative, dFFLIPTAT.
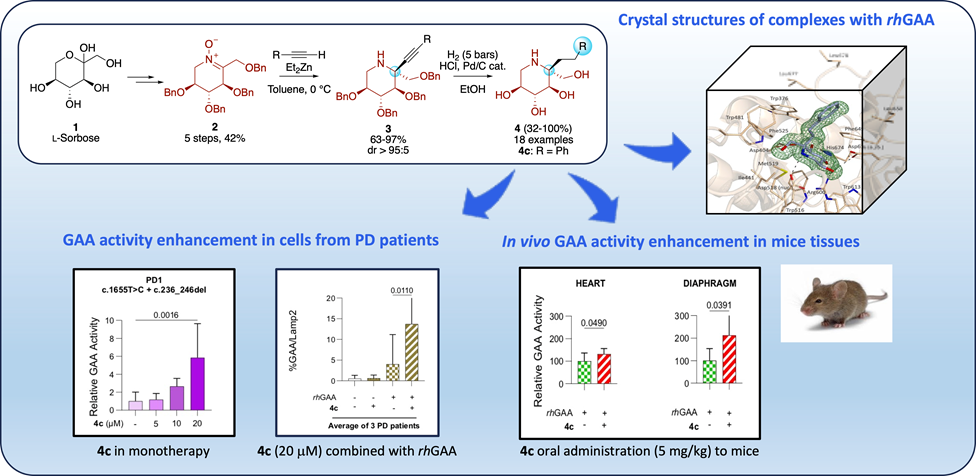
We report herein the design and synthesis of a series of 5-C-alkyldeoxynojirimycins from l-sorbose, through an efficient and scalable method amenable to preparing a large variety of analogues. The interaction of this class of compounds with human acid α-glucosidase (GAA), the genetically defective enzyme in patients suffering from Pompe disease, was investigated to identify pharmacological chaperones exhibiting high selectivity for this enzyme. Crystallographic analyses provided a rationale for their binding mode to GAA and chaperone activity. The effects of 5-C-phenethyl-DNJ (4c) were evaluated on GAA activity enhancement in cells from Pompe disease patients and in vivo in GAA-KO mice. The significant increase of GAA activity in the presence of 4c in various tissues, particularly in the diaphragm, encourages further studies on this class of small molecules toward developing clinical drugs. Their chaperone activity and excellent selectivity may offer potential benefits over the current treatments for Pompe disease.
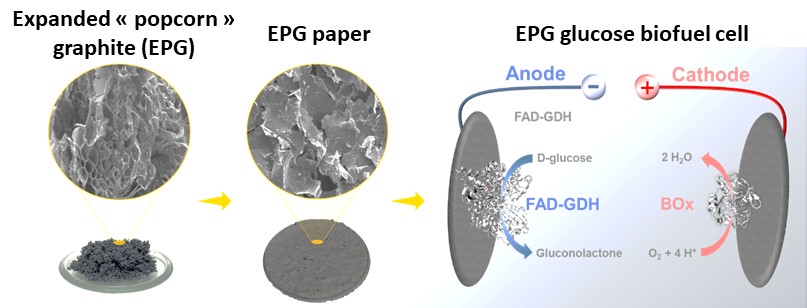
Here, we demonstrate the beneficial use of expanded graphite as an electrode material suitable for enzyme wiring for bioelectrocatalytic energy conversion. Expanded graphite is obtained via microwave treatment using commercial expandable graphite, which leads to a drastic popcorn-like volume increase. After an adjusted dispersion and filtration procedure, enzymatic bioanodes are formed using FAD-glucose dehydrogenase (FAD-GDH) with 9,10 phenanthroline-5,6-dione (PLQ) as an electron shuttle. For the bioanode, a current density of 2.87 mA cm⁻² is obtained at 100 mmoL-1 glucose concentration at 0.3 V vs Ag/AgCl (sat. KCl). The biocathode provided a current density of -0.41 mA cm⁻² in oxygen saturated buffer solution at 0.2 V. These performances are comparable to lab-made CNT-BPs and commercial CNT-BPs. expanded graphite represents therefore a cost-efficient and non-toxic alternative to expensive and potentially toxic nanomaterials.
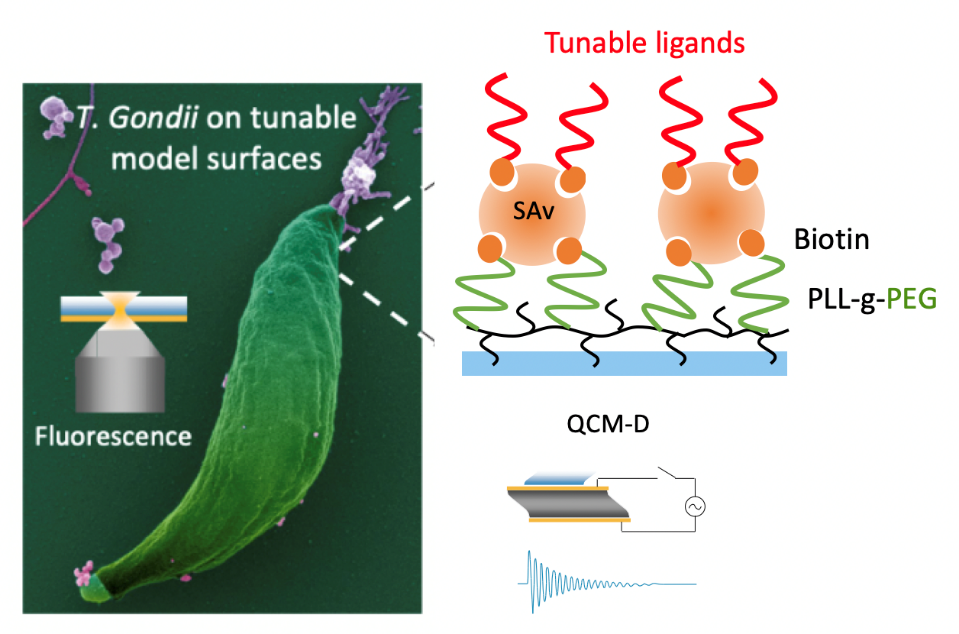
Toxoplasma adhesion strategy for gliding motility
DCM & IAB & LIPHY
Combining tunable surface chemistry and micropatterning with flow force and super-resolution imaging, we showed that Toxoplasma gondii (T. gondii) parasites build only one apical anchoring contact with the substrate, over which they slide. Furthermore, we showed that glycosaminoglycan(GAG)–parasite interactions are sufficient to promote such force-productive contact and found that the apicobasal flow is set up independent of adhesin release and surface interactions. This study is on-going to understand the role of other interfacial parameters in the T. gondii motility, including surface density and lateral mobility of the GAG ligands (supported by ANR PRC "MiniToxoAd", IAB/DCM). The developed chemical and biological tools should enable further characterization of the T. gondii–substrate interface, including their invasion ability, and its comparison across different parasites.
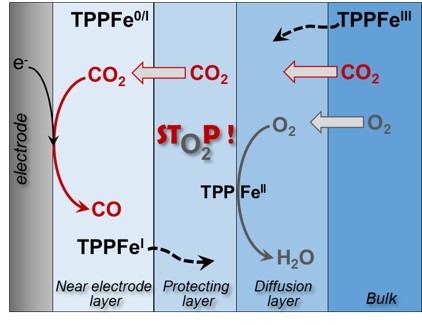
The electrochemical reduction of CO2, coupled with renewable energy, offers a promising approach to convert CO2 to valuable products. A critical challenge for practical application is achieving O2 tolerance─the ability of the catalyst to sustain CO2 reduction without degradation in the presence of O2. This study highlights the self-protection mechanism of TPPFe in homogeneous electrocatalysis against O2 and reactive oxygen species (ROS). Using rotating disk voltammetry, constant potential electrolysis, and spectro-electrochemistry, we demonstrate that lesser reduced TPPFe states selectively reduce O2, form a protective layer that shields the active catalyst for CO2 reduction. This self-protection mechanism, applicable to other catalysts with multiple redox states and adaptable to molecular catalysts immobilized in thick films, underscores the importance of optimizing mass transport conditions and catalyst design to achieve an O2-tolerant CO2 reduction.
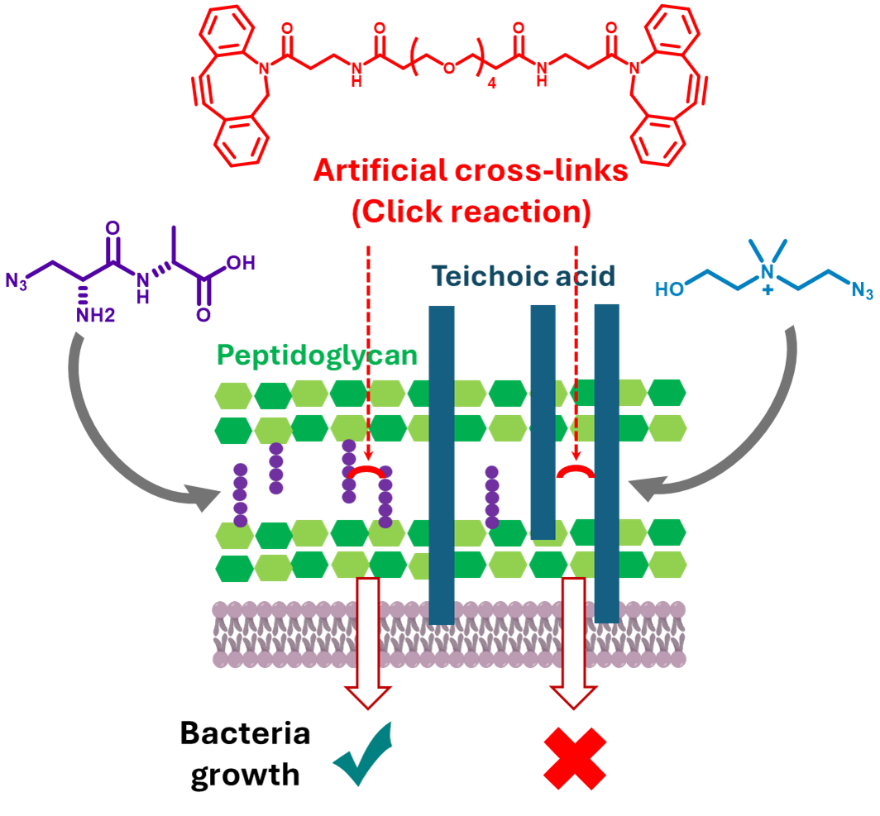
This work identifies a novel antibacterial mechanism that targets the cell wall of the human pathogen Streptococcus pneumoniae. Unlike conventional cell-wall targeting antibiotics, which inhibit the natural cross-linking of peptidoglycan, we introduce artificial cross-links using metabolic engineering to insert a clickable function into the other main component of the Gram-positive cell wall, the teichoic acids, then perform Strain-Promoted Alkyne-Azide Cycloaddition (SPAAC) and demonstrate that this results in impaired cell growth.
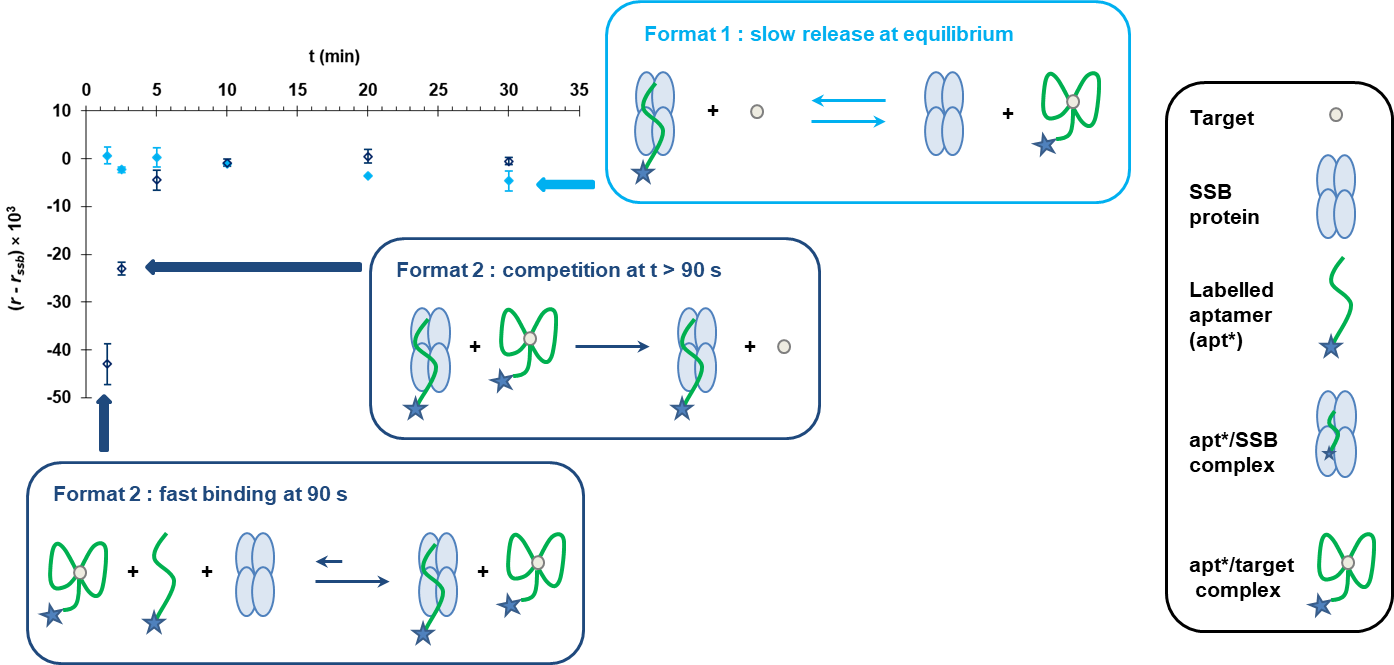
Due to their ability to bind aptamers in the free rather than in the target-bound folded state, single-stranded DNA-binding (SSB) proteins hold great promise in bioanalysis. SSB reagents are usually exploited through the target-induced displacement of aptamer pre-bound to the protein. However, the interaction strength between DNA molecules and SSB is intrinsically associated with lengthy desorption that (i) limits the range of application to oligonucleotides that do not associate too strongly with the protein and (ii) hampers the speed of the assays. Herein, we applied a very simple approach to solve this problem. Rather than working on the typical design of DNA displacement, we implemented pre-incubation of the target and aptamer before adding SSB to reveal the unbound aptamer fraction. We focused on the free-state unstructured anti-L-tyrosinamide (L-Ym) DNA aptamer, which was particularly relevant for the proof-of-concept study due to its potential to tightly bind to SSB with very slow off-rates. We considered fluorescence anisotropy (FA) for monitoring changes in the binding reactions of fluorescently labelled aptamer upon L-Ym addition. While no FA response was attainable under conventional displacement conditions, our approach enabled to generate substantial target-dependent FA signal in just 90 s. Under optimised conditions, we were able to achieve a detection threshold as low as 700 pM, which is the lowest ever documented for L-Ym in homogeneous-phase assays. We believe that the present scheme will enable SSB-based aptamer assays and sensors to greatly expand their scope of practical application.
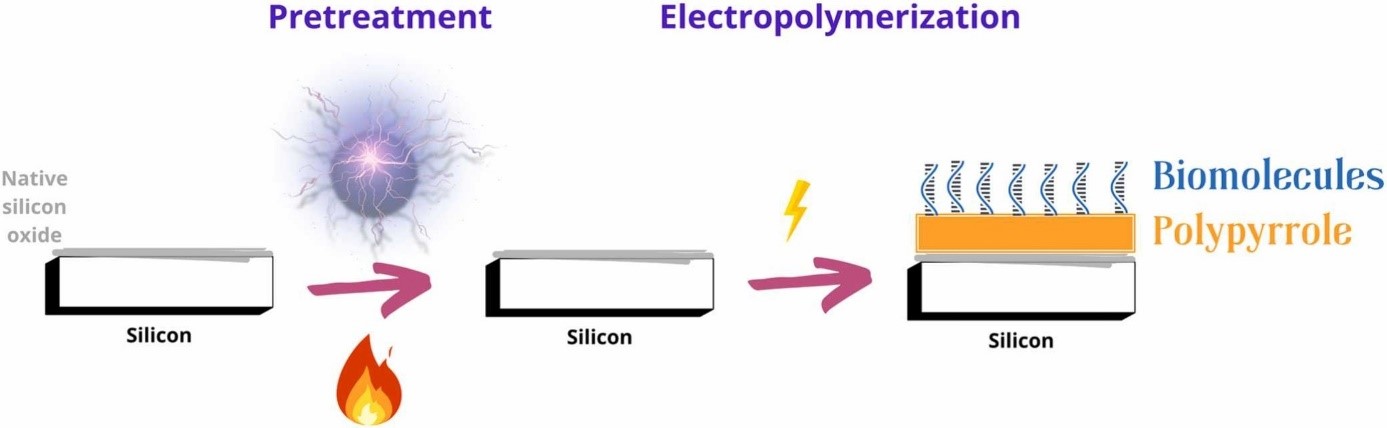
Ultrathin polypyrrole films were electropolymerized directly onto native or minimally treated silicon in a single step. Film thickness, morphology, and oxidation state were tuned by varying pretreatment, deposition and post-treatment conditions. The method supports localized electrospotting in microliter volumes for multiplexed biofunctionalization and could be adapted in the future to biosensing and micro/nanofluidics platforms needing surface functionality and charge control.
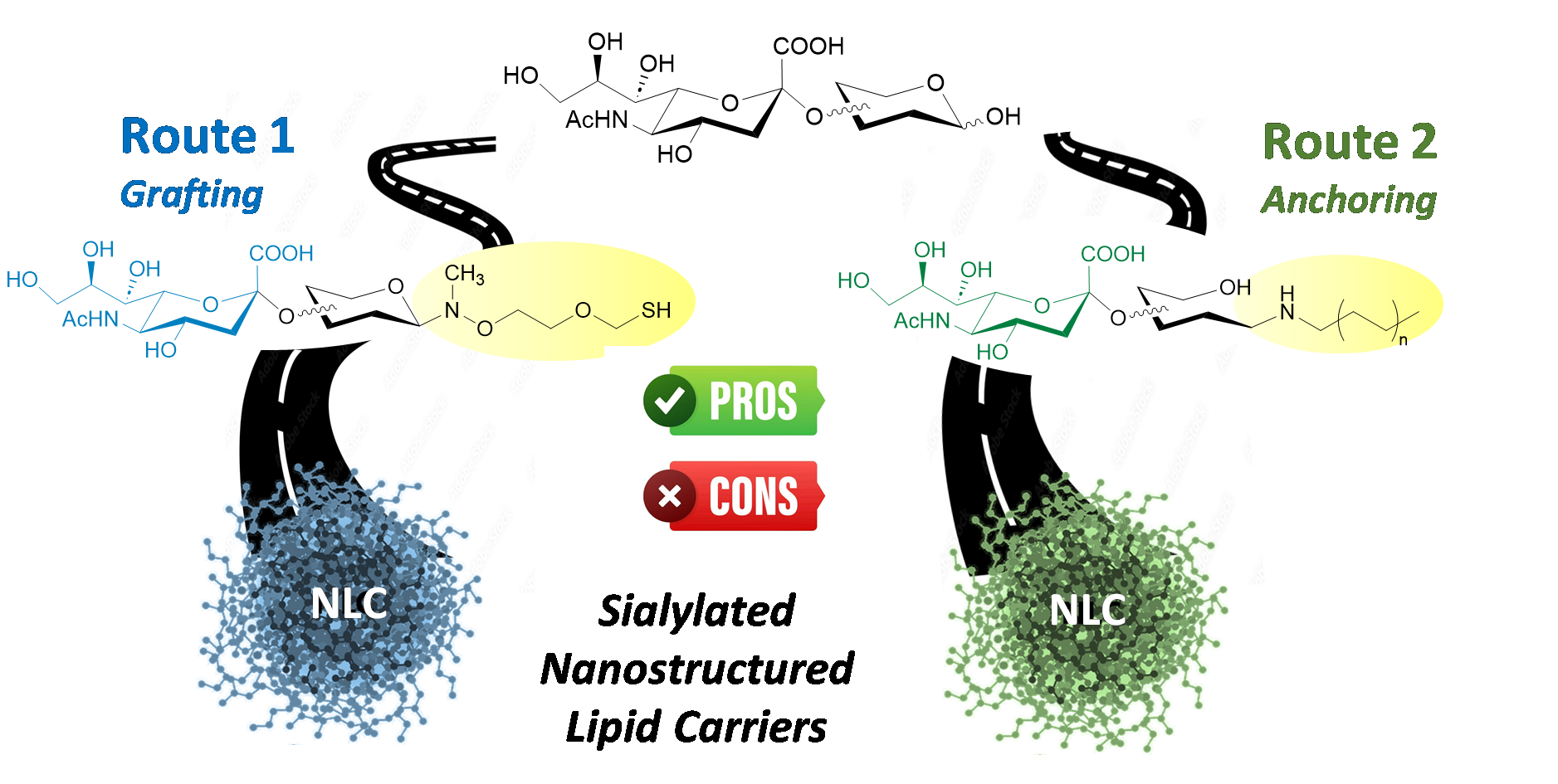
Nanostructured lipid carriers (NLCs) have emerged as a promising platform for drug delivery, offering advantages such as high drug-loading capacity and improved physicochemical stability. To enhance their targeting specificity, surface functionalization is crucial, as it enables selective interactions with biological receptors. In this study, we evaluated two distinct chemical strategies for the glycoengineering of NLCs. The grafting method proved superior, yielding glycosylated NLCs that were stable, biocompatible, and efficiently functionalized.
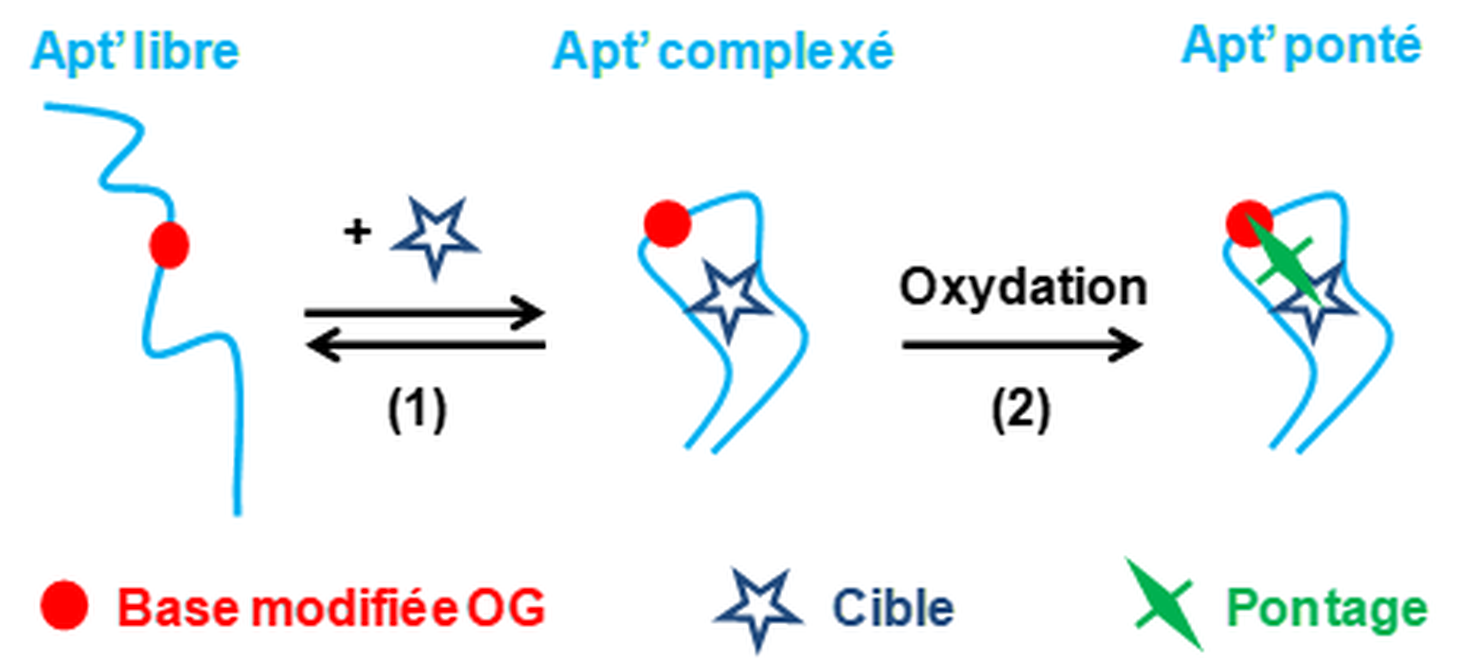
Aptamers-target crosslinks
Symmes & DPM
The effect of the substitution of a guanine base by the oxidative lesion 8-oxo-7,8-dihydroguanine (OG) on the affinity of the DNA aptamer selected against L-argininamide (L-Rm) was studied by fluorescence anisotropy. Results shown that, depending on the OG position, the substitution either reduces, does not affect or increases the affinity. Specific oxidation of the OG-containing aptamers by an Ir(IV) salt was carried out to promote crosslinks formation with the L-Rm target. Results shown that crosslinks occur with the OG-containing aptamers but also with scramble sequences not supposed to bind L-Rm. Efforts to reduce formation of such unspecific crosslinks were only partially successful, as they failed to demonstrate that part of these adducts originated from specific recognition of aptamers towards L-Rm.
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn