DPM & SyMMES
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
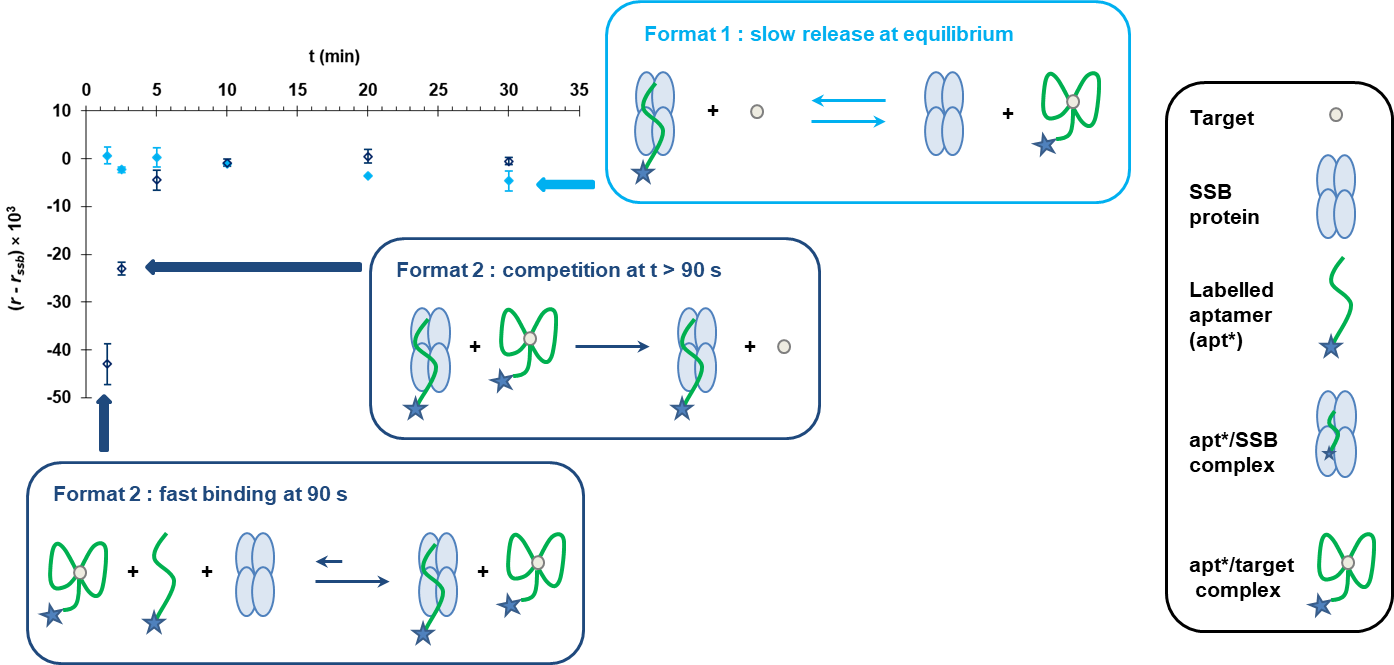
Due to their ability to bind aptamers in the free rather than in the target-bound folded state, single-stranded DNA-binding (SSB) proteins hold great promise in bioanalysis. SSB reagents are usually exploited through the target-induced displacement of aptamer pre-bound to the protein. However, the interaction strength between DNA molecules and SSB is intrinsically associated with lengthy desorption that (i) limits the range of application to oligonucleotides that do not associate too strongly with the protein and (ii) hampers the speed of the assays. Herein, we applied a very simple approach to solve this problem. Rather than working on the typical design of DNA displacement, we implemented pre-incubation of the target and aptamer before adding SSB to reveal the unbound aptamer fraction. We focused on the free-state unstructured anti-L-tyrosinamide (L-Ym) DNA aptamer, which was particularly relevant for the proof-of-concept study due to its potential to tightly bind to SSB with very slow off-rates. We considered fluorescence anisotropy (FA) for monitoring changes in the binding reactions of fluorescently labelled aptamer upon L-Ym addition. While no FA response was attainable under conventional displacement conditions, our approach enabled to generate substantial target-dependent FA signal in just 90 s. Under optimised conditions, we were able to achieve a detection threshold as low as 700 pM, which is the lowest ever documented for L-Ym in homogeneous-phase assays. We believe that the present scheme will enable SSB-based aptamer assays and sensors to greatly expand their scope of practical application.
Extending the range of SSB-assisted aptamer assays to functional DNA with very tight protein binding, Auriane Guitton Auberty, Eric Peyrin, Jérémy Nenadovic, Jean-Luc Ravanat, Sandrine Perrier, Sensors and Actuators B: Chemical, 2025, 437, 137725
Arcane PhD student
Auriane Guitton Auberty
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn