CERMAV & ILL
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
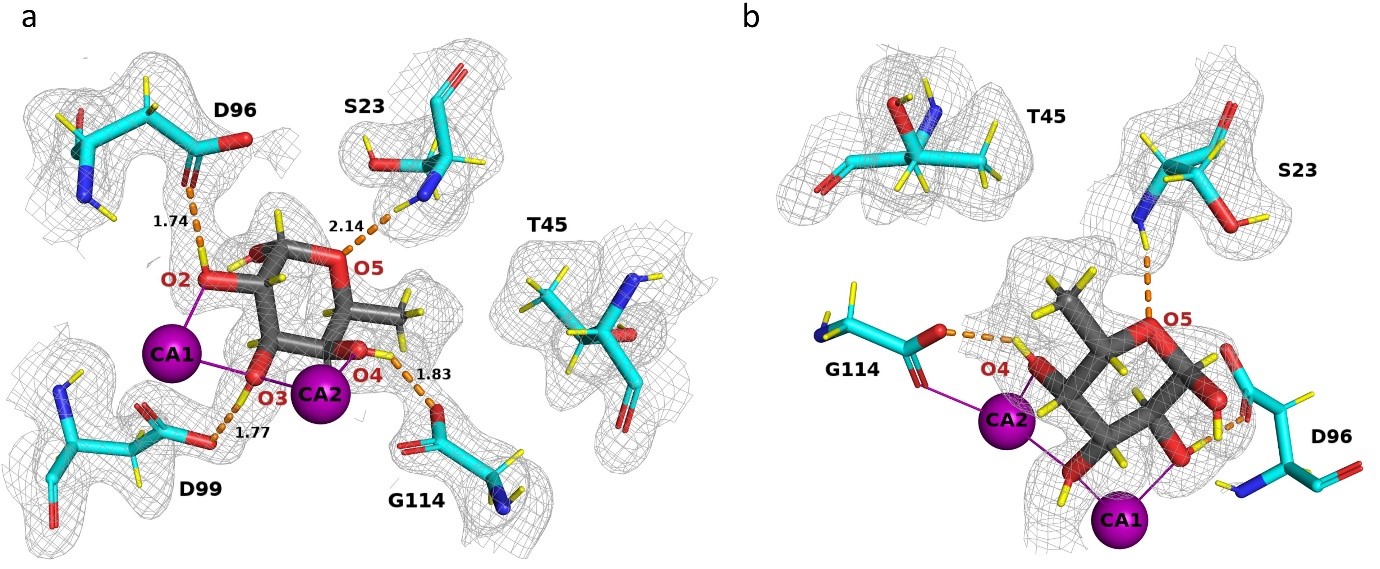
In view of the threat to human health caused by bacterial antibiotic resistance, new strategy, such as anti-infectious compounds, are of high interest. Pseudomonas aeruginosa, a major cause of nosocomial infections, uses carbohydrate-binding proteins (lectins) as part of its binding to host cells. A soluble fucose-binding lectin, LecB, displays a unique carbohydrate-binding site that involves two closely located calcium ions bridging between the ligand and protein, providing specificity and unusually high affinity. Several high resolution X-ray crystal structures of LecB/fucose complex are available. However, the location of hydrogen atoms, that are crucial in the interaction mechanisms are not visible by X-ray crystallography.
Neutron crystallography was therefore used to localize hydrogen atoms directly in the structure. To gain as much information as possible from the neutron diffraction experiments, both the protein and the sugar were produced in deuterated forms with all hydrogen atoms replaced by deuterium atoms. Data collection was performed at room temperature, close to physiological conditions. The structure of the complex provided an outstanding level of detail which would not have been possible using X-ray crystallography alone. A complete hydrogen-bond network could be observed between the protein and its ligand. A low-barrier hydrogen bond was also identified a special type of strong hydrogen bond which plays a role in the unusually high affinity of LecB with fucose.
The unusually high affinity of LecB for fucose and fucosylated oligosaccharides can be rationalized by a synergy between short hydrogen bonds, including a low-barrier one, and coordination bonds between the sugar, protein and calcium ions and two water molecules. Moreover, the binding of fucose causes displacement of other tightly-bound water molecules that are present in the sugar-free structure into the bulk solvent that is accompanied by a favorable entropy of binding. Both the high enthalpic contributions from strong hydrogen bonds, a favorable entropic term, together with charge delocalization caused by the involvement of two close calcium ions, play a role in the high affinity. From RT neutron diffraction data, it can be observed that water molecules which participate in the sugar binding display high mobility. The information presented here will be important in rational structure-based drug design of potent inhibitors of Pseudomonas aeruginosa. It is believed that this work and these types of approaches will make important contributions to the development of glycomimetics that could help deal with the resurgence of multi-drug resistance bacteria.
Neutron crystallography reveals mechanisms used by Pseudomonas aeruginosa for host-cell binding, L. Gajdos, M.P. Blakeley, M. Haertlein, V. T. Forsyth, J. M. Devos & A. Imberty, Nature Communications, 2022, 13, 194.
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn