DCM
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
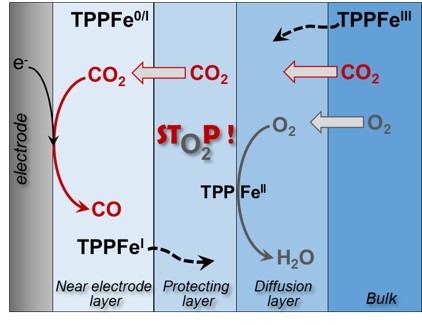
The electrochemical reduction of CO2, coupled with renewable energy, offers a promising approach to convert CO2 to valuable products. A critical challenge for practical application is achieving O2 tolerance─the ability of the catalyst to sustain CO2 reduction without degradation in the presence of O2. This study highlights the self-protection mechanism of TPPFe in homogeneous electrocatalysis against O2 and reactive oxygen species (ROS). Using rotating disk voltammetry, constant potential electrolysis, and spectro-electrochemistry, we demonstrate that lesser reduced TPPFe states selectively reduce O2, form a protective layer that shields the active catalyst for CO2 reduction. This self-protection mechanism, applicable to other catalysts with multiple redox states and adaptable to molecular catalysts immobilized in thick films, underscores the importance of optimizing mass transport conditions and catalyst design to achieve an O2-tolerant CO2 reduction.
C. M. Harvey, S. Chardon-Noblat, C. Costentin*
J. Am. Chem. Soc., 2025, 147, 24171-24178
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn