DPM & DCM
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
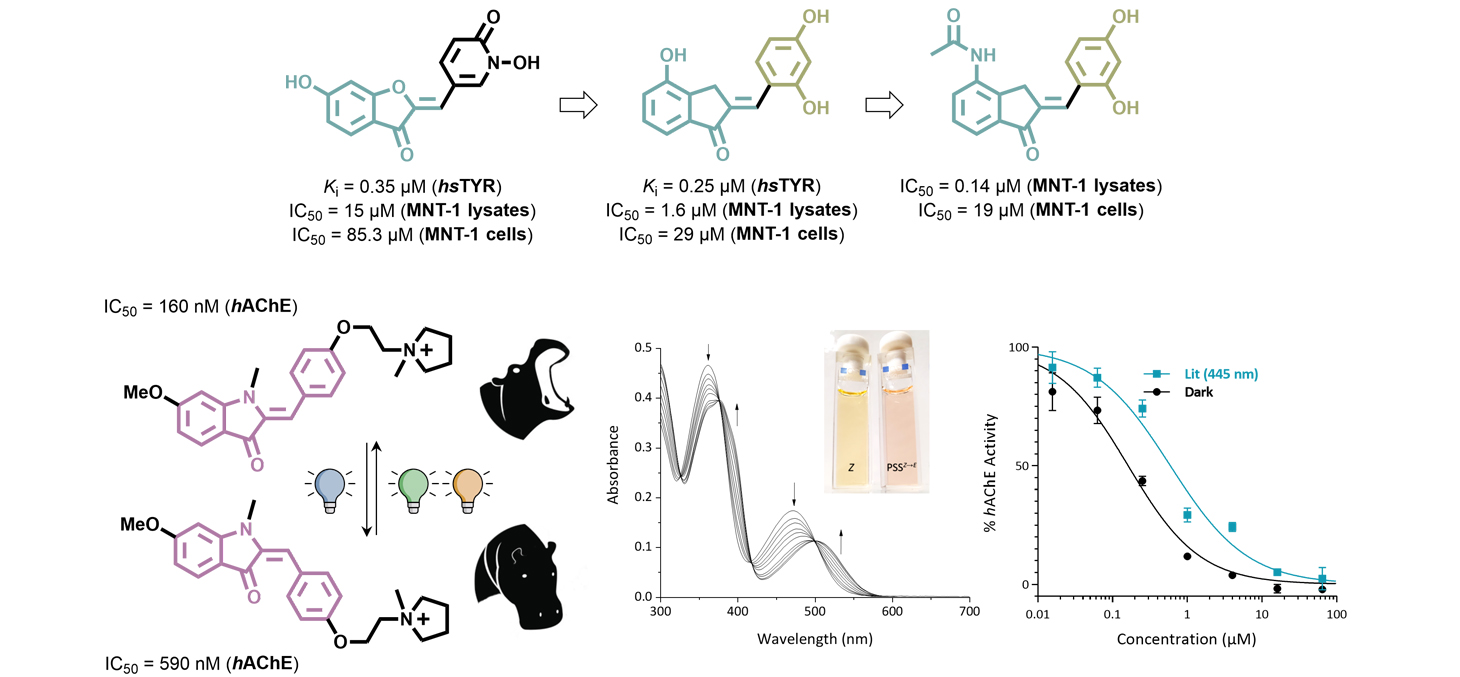
The project enabled the development of new series of hemi-indigoid compounds targeting two distinct applications. In the first line of research, three successive generations of human tyrosinase inhibitors were synthesized, yielding over one hun-dred distinct compounds. These inhibitors demonstrated sub-micromolar activity and exhibited enhanced efficacy in cellular models, highlighting their potential for therapeutic applica-tions. In the second line of work, eight photoswitchable hemi-indigoid derivatives were designed and synthesized as the first reported photopharmacological compounds of this class. These compounds functioned as acetylcholinesterase inhibitors, of-fering a novel approach for visible-light-controlled modulation of enzyme activity. Together, these efforts underscore the versatility of hemiindigoid scaffolds for both enzymatic inhibi-tion and photopharmacological applications.
L. M. Lazinski, G. Royal, M. Robin, M. Maresca, R. Haudecoeur. J. Med. Chem. 2022, 65, 12594–12625
B. Roulier, I. Rush, L. M. Lazinski, B. Pérès, H. Olleik, G. Royal, A. Fishman, M. Maresca, R. Haudecoeur. Eur. J. Med. Chem. 2023, 246, 114972.
M. Beaumet, L. M. Lazinski, M. Maresca, R. Haudecoeur. Eur. J. Med. Chem. 2023, 259, 115672.
L. M. Lazinski, M. Beaumet, B. Roulier, R. Gay, G. Royal, M. Maresca, R. Haudecoeur. Eur. J. Med. Chem. 2024, 266, 116165.
M. Beaumet, L. M. Lazinski, M. Maresca, R. Haudecoeur. ChemMedChem 2024, 19, e202400314.
L. M. Lazinski, M. Beaumet, F. Loiseau, C. Goudet, M. Boggio-Pasqua, G. Royal, R. Haudecoeur. ChemistryEurope 2025, 3, e202500221.
M. Beaumet, L. M. Lazinski, B. Roulier, G. Royal, M. Maresca, R. Haudecoeur. Submitted.
Arcane PhD student
Leticia da Mata LAZINSKI CIVIL
Supervisors
Romain HAUDECOEUR (DPM) & Guy ROYAL (DCM)
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn